Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Ozawa, Masaki; Iwai, Takehiko*; Babain, V.*; Shadrin, A.*
Proceedings of International Solvent Extraction Conference "Solvent Extraction-Fundamentals to Industrial Applications" (ISEC 2008), p.623 - 628, 2008/09
Bifunctional organophosphorus extractants dissolved in polar fluorinated diluents were studied aiming at directly recovering all f-elements from the dissolver solution of spent nuclear fuel. Distribution ratios of U, Np and Pu were sufficiently high for 0.4 0.8MCMPO in this solvent system, and combination of salt-free, methylamine carbonate (MAC), etc, were evaluated to obtain fractional stripping of f-elements. Static multi-stage extraction using artificial FBR dissolver solution supported the process feasibility.
0.8MCMPO in this solvent system, and combination of salt-free, methylamine carbonate (MAC), etc, were evaluated to obtain fractional stripping of f-elements. Static multi-stage extraction using artificial FBR dissolver solution supported the process feasibility.
Matsumura, Tatsuro; Inaba, Yusuke*; Takeshita, Kenji*; Mori, Atsunori*
Proceedings of International Solvent Extraction Conference "Solvent Extraction-Fundamentals to Industrial Applications" (ISEC 2008), 4 Pages, 2008/09
We are developing a new MA/Ln separation process with derivatives of TPEN (N,N,N',N'-tetrakis(2-pyridylmethyl)ethylenediamine) for P&T technology. TPEN has good selectivity of Am(III) from Ln(III). However, TPEN is soluble slightly (about 10 mol/l) to water. In this study, the hydrophobicity of TPEN is improved by introducing alkyl groups and the selectivity of Am(III) and Eu(III) is examined. We succeeded to synthesize several new hydrophobic derivative of TPEN. One of the ligands, TDdPEN (N,N,N',N'-tetrakis((5-dodecyloxypyridin-2-yl)-methyl)ethylenediamine) showed high selectivity. The maximum separation factor, SF
mol/l) to water. In this study, the hydrophobicity of TPEN is improved by introducing alkyl groups and the selectivity of Am(III) and Eu(III) is examined. We succeeded to synthesize several new hydrophobic derivative of TPEN. One of the ligands, TDdPEN (N,N,N',N'-tetrakis((5-dodecyloxypyridin-2-yl)-methyl)ethylenediamine) showed high selectivity. The maximum separation factor, SF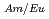 , was over 800. Hydrophobic derivatives of TPEN that have potential of application to the MA/Ln separation process was synthesized successfully.
, was over 800. Hydrophobic derivatives of TPEN that have potential of application to the MA/Ln separation process was synthesized successfully.
Sasaki, Yuji; Kitatsuji, Yoshihiro; Hirata, Masaru; Kimura, Takaumi; Yoshizuka, Kazuharu*
Proceedings of International Solvent Extraction Conference "Solvent Extraction-Fundamentals to Industrial Applications" (ISEC 2008), p.745 - 750, 2008/09
The multidentate diamides are synthesized and examined for the actinide (An) extractions, bi- and tridentate extractants are focused in this work. The extraction of actinide was performed from 0.1-6M HNO to organic solvents. It was obvious that tetraalkyl-diglycolamide (DGA) derivatives, (methylimino)bis(N,N-dioctylacetamide) (MIDOA) and dimethyl-dioctyl-2-(3-oxapentadecane)-malonamide (DMDOOPDMA) have relatively high D values (D(Pu): more than 70). The following notable results using DGA extractants are obtained, (1) DGA with short alkyl chain give higher D values than those with long alkyl chain, (2) DGA with long alkyl chain have high solubility in n-dodecane. The molecular modeling with computational chemistry was also used to elucidate the effects of structural and electronic properties of the reagents on their different extractabilities.
to organic solvents. It was obvious that tetraalkyl-diglycolamide (DGA) derivatives, (methylimino)bis(N,N-dioctylacetamide) (MIDOA) and dimethyl-dioctyl-2-(3-oxapentadecane)-malonamide (DMDOOPDMA) have relatively high D values (D(Pu): more than 70). The following notable results using DGA extractants are obtained, (1) DGA with short alkyl chain give higher D values than those with long alkyl chain, (2) DGA with long alkyl chain have high solubility in n-dodecane. The molecular modeling with computational chemistry was also used to elucidate the effects of structural and electronic properties of the reagents on their different extractabilities.